.png)
Solid State
Energy Conversion
Devices
About Us
The research interests of Skinner research group centre on the properties and structures of ion-conducting oxides, with emphasis on the identification and characterisation of new materials using in- situ high-temperature techniques such as x-ray and neutron powder diffraction techniques, secondary ion mass spectrometry and low energy ion scattering. This work has potential applications in the development of solid oxide fuel cell, electrolysis and permeation membranes and more have been identified as having application in the field of novel solid-state gas sensors.
Our Latest Publications
Here, we investigated the high temperature structural properties of the LaNb1–xWxO4+d (x = 0.04–0.16) family, which is a structural analogue of hyper-stoichiometric CeNbO4+d.

The importance of A-site cation chemistry in superionic halide solid electrolytes

Halide solid electrolytes do not currently display ionic conductivities suitable for high-power all-solid-state batteries. We explore the model system A2ZrCl6 (A = Li, Na, Cu, Ag) to understand the fundamental role that A-site chemistry plays on fast ion transport. Having synthesised the previously unknown Ag2ZrCl6 we reveal high room temperature ionic conductivities in Cu2ZrCl6 and Ag2ZrCl6 of 1 × 10^−2 and 4 × 10^−3 S cm^−1, respectively. We introduce the concept that there are inherent limits to ionic conductivity in solids, where the energy and number of transition states play pivotal roles. Transport that involves multiple coordination changes along the pathway suffer from an intrinsic minimum activation energy. At certain lattice sizes, the energies of different coordinations can become equivalent, leading to lower barriers when a pathway involves a single coordination change. Our models provide a deeper understanding into the optimisation and design criteria for halide superionic conductors.

Revealing Local Grain Boundary Chemistry and Correlating it with Local Mass Transport in Mixed-Conducting Perovskite Electrodes

Grain boundary (GB) mass transport, and chemistry exert a pronounced influence on both the performance and stability of electrodes for solid oxide electrochemical cells. Lanthanum strontium cobalt ferrite (LSCF6428) is applied as a model mixed ionic and electronic conducting (MIEC) perovskite oxide. The cation-vacancy distribution at the GBs is studied at both single and multi-grain scales using high-resolution characterization techniques and computational approaches. The accumulation of oxygen vacancies in the GB region, rather than necessarily at the GB core, results in an enhancement of the oxygen diffusivity by 3 – 4 orders of magnitude along the GBs. The origin of the concomitant changes in the cation composition is also investigate. In addition to the host cations, strong Na segregation is detected at all the GBs examined. Despite the low (ppm) level of this impurity, its presence can affect the space charge potential (Φ0). This, in turn, will influence the evolution of GB chemistry.

Thermal stability and coalescence dynamics of exsolved metal nanoparticles at charged perovskite surfaces
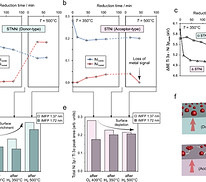
Exsolution reactions enable the synthesis of oxide-supported metal nanoparticles, which are desirable as catalysts in green energy conversion technologies. It is crucial to precisely tailor the nanoparticle characteristics to optimize the catalysts’ functionality, and to maintain the catalytic performance under operation conditions. We use chemical (co)-doping to modify the defect chemistry of exsolution-active perovskite oxides and examine its influence on the mass transfer kinetics of Ni dopants towards the oxide surface and on the subsequent coalescence behavior of the exsolved nanoparticles during a continuous thermal reduction treatment. Nanoparticles that exsolve at the surface of the acceptor-type fast-oxygen-ion-conductor SrTi0.95Ni0.05O3−δ (STNi) show a high surface mobility leading to a very low thermal stability compared to nanoparticles that exsolve at the surface of donor-type SrTi0.9Nb0.05Ni0.05O3−δ (STNNi). Our analysis indicates that the low thermal stability of exsolved nanoparticles at the acceptor-doped perovskite surface is linked to a high oxygen vacancy concentration at the nanoparticle-oxide interface. For catalysts that require fast oxygen exchange kinetics, exsolution synthesis routes in dry hydrogen conditions may hence lead to accelerated degradation, while humid reaction conditions may mitigate this failure mechanism.
